Epigenetics: Identifying Factors Governing Cellular Identity
Epigenetics plays a crucial role in orchestrating gene expression patterns during development and disease. We are dedicated to identifying specific chromatin factors that shape epigenetic landscapes of cells. We construct and use focused CRISPR libraries to interrogate the function of chromatin factors in reprogramming, differentiation, transformation into malignant cells and acquisition of drug resistance. Through a combination of functional genomics and genome-wide we aim to decipher the language of epigenetic modifications and their impact on gene regulation. Our findings not only contribute to the fundamental understanding of epigenetic mechanisms but also hold potential for therapeutic interventions targeting epigenetic dysregulation in various disease contexts
Click on epigenetics in the style of Monet for relevant article.
Chemical Reprogramming: Unlocking Cellular Potential
One of our primary research areas is chemical reprogramming, an emerging field that explores the ability to manipulate cell identity and fate using small molecules. By understanding the underlying mechanisms that govern cellular reprogramming, we aim to develop novel strategies for tissue regeneration, disease modeling, and therapeutic interventions. Our team’s expertise lies in identifying key molecular drivers and epigenetic modifiers that enable the conversion of one cell type to another, opening new frontiers in regenerative medicine.
Click on stem cells in the style of Jackson Pollock for relevant article.

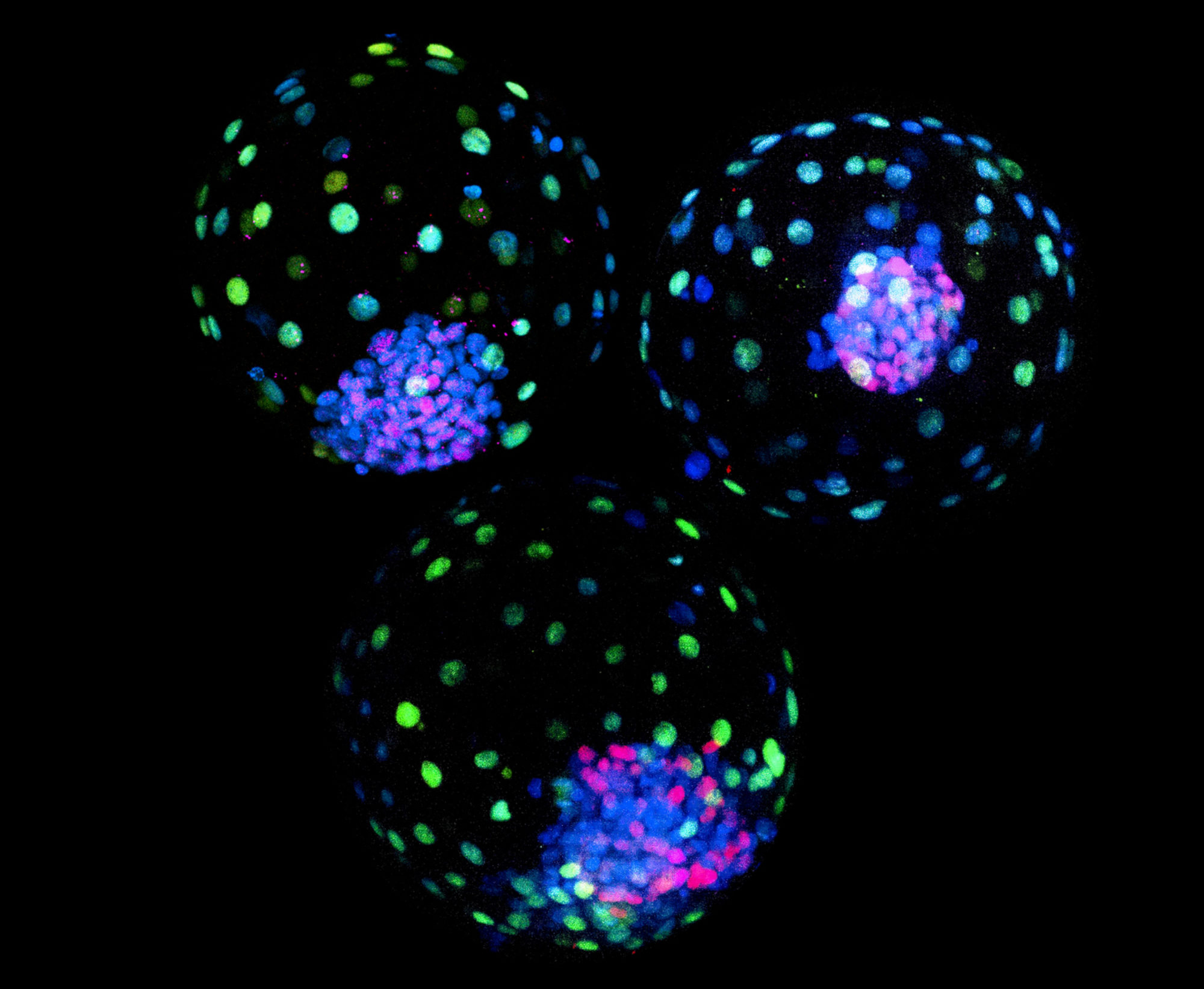
Early Embryonic Development: Decoding the Blueprint of Human Embryogenesis
The earliest stages of embryonic development are a series of intricate events that lay the foundation for complex organisms. We are fascinated by this remarkable process and aim to uncover the underlying transcriptional and epigenetic cues that guide embryogenesis. By using CRIPSR-based genome editing, reporter stem cell lines and in vitro blastoid models, we aim to shed light on the dynamic interplay between transcriptional regulation, epigenetic modifications, and cellular signaling during early development. Our research has the potential to revolutionize our understanding of human development and provide valuable insights into congenital disorders and pregnancy-related complications.
Click on the blastocyte in the style of Van Gogh for relevant article.
Stem Cell-based Disease Modelling: Generating Renewable Cell Sources
By reprogramming adult cells into a pluripotent state, iPSCs offer an unprecedented opportunity to generate patient-specific disease models in a laboratory setting. These models faithfully recapitulate the genetic and cellular characteristics of the disease, enabling us to investigate disease mechanisms, identify novel therapeutic targets, and test potential treatments in a controlled environment, ultimately guiding personalized medicine approaches. In our lab, we are generating iPSCs from patients with rare diseases so that we have a limitless source of cells for research, eliminating ethical concerns associated with embryonic stem cells and allowing us to study such rare and inaccessible diseases.
Click on the disease cells in the style of Picasso for relevant article.